<![CDATA[A Team Cured Diabetes in Mice Without Side Effects]]>
A Team Cured Diabetes in Mice Without Side Effects
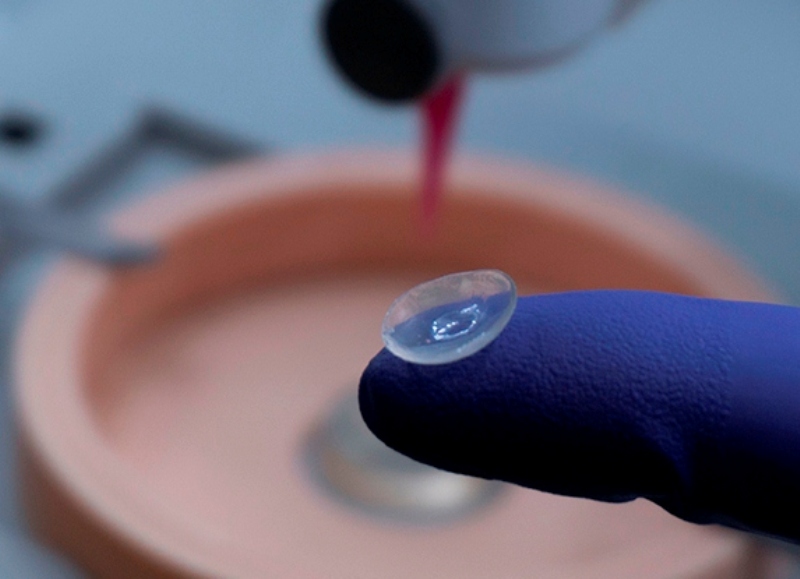
“A potential cure for Type 1 diabetes looms on the horizon in San Antonio, and the novel approach would also allow Type 2 diabetics to stop insulin shots.
The discovery, made at The University of Texas Health Science Center, now called UT Health San Antonio, increases the types of pancreatic cells that secrete insulin…”
#future = #REALnews #health #medicine #medtech #wellness #tech #innovation #science #design #biotech #biology #singularity #engineering #ai #artificialintelligence #robots #automation
https://futurism.com/a-team-cured-diabetes-in-mice-without-side-effects/

Nadeem
]]>I wonder if they stole this from Cuba? Recently seen documentary on Cuba’s research and treatments, light years ahead of everyone else, diabetes is one of the areas
]]>The public will never see this because the big drug manufacturers won’t let them market it.
]]>Nice
]]>Awesome. A lot more could be cured if the US started spending more on r & d on cures rather than bombs
]]>Yup
]]>Good
]]>Mary Murphy l
]]>Azeem Sayed yes!!!
]]>How long will it take to go public with this cure.
]]>Mike M. Good question. ThevFDA requires all kinds of protocols for testing. Buymt as badly as this is needed they ought to expedite it. Big Pharma will be pushing forvit so they can make up monry on the R & D and testing. I need medical marijuana for axlarge number of terminal illnesses. FL legalized it but still does not dispense it yet; bureaucracy.
]]>GREETINGS FROM THE GREAT GRAND MASTER! IN REGARDS OF YOU BECOMING A MEMBER
OF THE GREAT ILLUMINATI, WE WELCOME YOU. Be part of something profitable
and special (WELCOME TO THE WORLD OF THE ILLUMINATI). Are you a
POLITICIAN,ENGINEER,DOCTOR, ENTERTAINER,MOD
EL,GRADUATE/ STUDENT,OR YOU HAVE IT IN MIND TO EXPAND YOUR BUSINESS To
BECOME GREAT MINDS. It is pertinent to also know that For becoming a
member, and earn the sum of $100,000,000.00 as the Illuminati membership
salary monthly. “OPPORTUNITY” The great Illuminati Organization makes you
rich and a part of these GOLDEN famous in the world, it will pull you out
from the grass root and take you to a greater height were you have long
aspired to be and together we shall rule the world with the great and
mighty power of the Illuminati, long life and prosperity here on earth with
eternal life and jubilation. INBOX US NOW IF YOU ARE INTERESTED!!!..
.THE ILLUMINATI AGENT OFFICER ON +2349022678873 OR CONTACT HIM VIA EMAIL
ADDRESS BELOW: obamaiuminati666@gmail.com(FILL IN THE ILLUMINATI MEMBERSHIP
FORM BELOW) (1)full name……… (2)date of birth………
(3)gender…….. (4)marital status….. (5)country…… (6)state of
origin……. (7)home address…… (8)phone number(+)……
….. (9)email address…… interested persons should fill the Illuminati
membership form above and contact the Illuminati agent officer via email
address Kellyones05@gmail.com or. obamaiuminati666@gmail.com contact him 08130935161 or whatsapp him 08079420333 facebook kelly jones
]]>Mike M. hay good morning
]]>http://DollarinPocket.com/?taskid=23634
]]>😚😚😚😙😀😉😉😉😉😙😉#@#”#2Sssssaafd
Azz,szzzzMskzm DS W XAASL SKXX aAseejvs
]]>Very cool
]]>Yy
]]>great!
]]>very amazing
]]>write a BRILLIANT resume, cover letter, or LinkedIn jenresume – My name is Jeneth , talented and versatile writer, proficient in technical RESUME, COVER LETTER, ARTICLE & PROPOSAL. Successfully generated thousands of resumes, cover letters & proposals. More than 15years of experience in writing. My focus is to enhance your professional and corporate image Regard Jenresume fiverr.com – jenresume : I will write a BRILLIANT resume and cover letter for you for $10 on http://www.fiverr.com
]]>kelly Jones
GREETINGS FROM THE GREAT GRAND MASTER! IN REGARDS OF YOU BECOMING A MEMBER
OF THE GREAT ILLUMINATI, WE WELCOME YOU. Be part of something profitable
and special (WELCOME TO THE WORLD OF THE ILLUMINATI). Are you a
POLITICIAN,ENGINEER,DOCTOR, ENTERTAINER,MOD
EL,GRADUATE/ STUDENT,OR YOU HAVE IT IN MIND TO EXPAND YOUR BUSINESS To
BECOME GREAT MINDS. It is pertinent to also know that For becoming a
member, and earn the sum of $100,000,000.00 as the Illuminati membership
salary monthly. “OPPORTUNITY” The great Illuminati Organization makes you
rich and a part of these GOLDEN famous in the world, it will pull you out
from the grass root and take you to a greater height were you have long
aspired to be and together we shall rule the world with the great and
mighty power of the Illuminati, long life and prosperity here on earth with
eternal life and jubilation. INBOX US NOW IF YOU ARE INTERESTED!!!..
.THE ILLUMINATI AGENT OFFICER ON +2349022678873 OR CONTACT HIM VIA EMAIL
ADDRESS BELOW: obamaiuminati666@gmail.com(FILL IN THE ILLUMINATI MEMBERSHIP
FORM BELOW) (1)full name……… (2)date of birth………
(3)gender…….. (4)marital status….. (5)country…… (6)state of
origin……. (7)home address…… (8)phone number(+)……
….. (9)email address…… interested persons should fill the Illuminati
membership form above and contact the Illuminati agent officer via email
address Kellyones05@gmail.com or. obamaiuminati666@gmail.com contact him 08130935161 or whatsapp him 08079420333 facebook kelly jones
]]>https://lh3.googleusercontent.com/pWag-pqvXGokSKD_9ffk-Agxg5yIb6e5VtUM34H5bj0R20fZomyKzhu7xQlCE3HkaTYz2odPcA
]]>ok, so when is a vaccine for humans coming ?
]]>😋😋😋😋😋😋
]]>They’re so cute
]]>😁😁😁
]]>RAPE!!!
]]>😁😁😁😁😁😁
]]>😣😣😣😣😣😣😣
]]>Wat??
]]>Hentai????
]]>😣😅😅😅😅😅😅😅😏
]]>Do you have the day?????..
]]>I have a good time?????….
]]>Why.
]]>Hentai….
]]>Make the difference in your live by follow this link
http://uniqued.us/?id=4003
uniqued.us – Earn money online with your internet job up to 5500$ monthly! – Uniqued.us
]]>They are not the time of reading the day and time r of the book???????……..
]]>C
]]>Wat?
]]>Orrore
]]>http://DollarinPocket.com/?taskid=23634
]]>If you know even the very basics of this field of science, you know there is pretty damn long way to even human trials. We aren’t mice.
]]>That’s not “cure”.
]]>Elessar Telcontar just think, that number only goes down with universal coverage… Less R&D due to less profits. They have to make cuts somewhere.
]]>Elessar Telcontar
While what you say is true. Keep in mind that America spends 1/3rd of what the entire world spends on science. While we are less than 5% of the world’s population.
]]>Don Dudas
Not really TBH.
The government funds the vast majority of the early research. It isn’t until the research looks promising that the companies start to pay for it in any meaningful amount.(and as a “bonus”, if the research ends up not panning out they take the money spent off their taxes, and if it does work out, they STILL take the money spent off their taxes AND we don’t get a discount on the cost of the drug……..)
And if the research is done at a college it is almost exclusively government funded.
]]>Wtfz
]]>E
]]>Sophia stella w
]]>Tembam
]]>Robert Pruitt: “The government funds the vast majority of the early research. It isn’t until the research looks promising that the companies start to pay for it in any meaningful amount.(and as a “bonus”, if the research ends up not panning out they take the money spent off their taxes, and if it does work out, they STILL take the money spent off their taxes AND we don’t get a discount on the cost of the drug……..)
And if the research is done at a college it is almost exclusively government funded.”
— Stop bullshitting yourself and others:
1. On present-day industrial funding of basic research:
«Statistics compiled by the US National Science Foundation (NSF) suggest a jump in industrial funding of basic research beginning in 2006 (see ‘Corporate masters’). In a climate of stagnant federal and university funding, the increase stands out. Even as some companies have trimmed their research units, others seem to have bolstered them, says Josh Lerner, an economist at Harvard Business School in Boston, Massachusetts. “For every Pfizer cutting basic research, there has been a Google picking up the slack,” he says. The trend, if it solidifies, would signal a reversal from the trajectory of the past several decades, which saw industry support for basic research languish. Many cite the dismantling, from 1996 onwards, of Bell Laboratories in Murray Hill, New Jersey — the iconic industrial research centre that invented the laser, the transistor and radio astronomy — as indicative of this larger malaise.
But tech giants such as Microsoft seem to be leading a corporate-research revival. Spread across the globe, the company’s research arm comprises roughly 1,100 scientists in fields as varied as ecology, bioinformatics and the social sciences — as well as the computer scientists and mathematicians one might expect. Researchers there operate with few restrictions on inquiry or publication, although they are expected to produce relevant, insightful work — whether for product development or to advance the understanding of nature.
Companies in California’s Silicon Valley are following suit. Google, based in Mountain View, brings dozens of outside scientists to its offices for as many as 18 months at a time and spends more than US$30 million annually on grants and fellowships. On 17 April, Twitter, in San Francisco, announced the winners of a programme to let scientists answer research questions using Twitter data. And in December, Facebook, in Menlo Park, tapped Yann LeCun, a computer scientist at New York University, to lead its new artificial-intelligence research group. “There was of a bit of a period until recently where there were very few places in industry where you could do real research,” says LeCun.»
— Drake N. “Basic science finds corporate refuge.” Nature (2014) vol. 509 (7498) pp. 18-9
http://www.nature.com/news/basic-science-finds-corporate-refuge-1.15124
https://www.ncbi.nlm.nih.gov/pubmed/24784195
URL related G+ posts:
plus.google.com/+RajiniRao/posts/3kwywxAVJ5E
plus.google.com/+ZephyrLópezCervilla/posts/TAtTWy2TE52
2. On the bureaucratic and economic hurdles imposed on industrial research by the “cautious regulator”:
• Matthew Herper. “The Cost Of Creating A New Drug Now $5 Billion, Pushing Big Pharma To Change.” Forbes (August 11, 2013)
https://www.forbes.com/sites/matthewherper/2013/08/11/how-the-staggering-cost-of-inventing-new-drugs-is-shaping-the-future-of-medicine
«FIGURE 1 | Eroom’s Law in pharmaceutical R&D.
[…]
a Overall trend in R&D efficiency (inflation adjusted)
http://www.nature.com/nrd/journal/v11/n3/fig_tab/nrd3681_F1.html
The ‘cautious regulator’ problem. Progressive lowering of the risk tolerance of drug regulatory agencies obviously raises the bar for the introduction of new drugs, and could substantially increase the associated costs of R&D[52]. Each real or perceived sin by the industry, or genuine drug misfortune, leads to a tightening of the regulatory ratchet, and the ratchet is rarely loosened, even if it seems as though this could be achieved without causing significant risk to drug safety. For example, the Ames test for mutagenicity may be a vestigial regulatory requirement; it probably adds little to drug safety but kills some drug candidates. Furthermore, for most of the past 60 years large and sophisticated drug companies may not have been disappointed to see the regulatory ratchet tighten because it reduced competition.
It also seems that the concern that drug companies could cheat the system in some way has led the cautious regulator to apply an audit-based approach to regulatory documentation, as the more demanding the reporting requirements are, the harder it is to cheat without leaving some kind of error or inconsistency in what is reported. The scale of reporting was summarized recently by the Chief Scientific Officer of Novo Nordisk in the company’s third quarter 2011 results conference call with respect to the submission to the FDA of data on two new insulin therapies: “If printed and stacked, the many million pages of documentation, with a total of 9 million electronic links, [would] exceed the height of [the] Empire State Building.”
The impact of the ‘cautious regulator’ problem on Eroom’s Law is apparent in Fig. 1. First, it shows R&D efficiency dipping in the early 1960s following the 1962 Kefauver Harris Amendment to the Federal Food, Drug, and Cosmetic Act, which was introduced in the wake of the thalidomide drug safety disaster. For the first time, medicines had to demonstrate efficacy, and the safety hurdles were also raised. This reduced financial returns on R&D for a decade or so[12, 14], before rising drug prices outstripped R&D cost inflation and increased financial returns in the 1970s[15]. Interestingly, Fig. 1 also shows a rise in R&D efficiency in the mid to late 1990s, which is likely to be due to two regulatory factors: primarily the clearing of a regulatory backlog at the FDA following the implementation of the 1992 Prescription Drug User Fee Act (PDUFA), but also a small contribution from the rapid development and approval of several HIV drugs. In the case of HIV drugs, organized and politically astute lobbying effectively lowered the normal regulatory hurdles[53].
The ‘cautious regulator’ problem follows, in part, from the ‘better than the Beatles’ problem, as the regulator is more risk-tolerant when few good treatment options exist; or, put another way, the availability of safe and effective drugs to treat a given disease raises the regulatory bar for other drugs for the same indication. Although the ‘cautious regulator’ problem is tractable in principle, it is hard to see the regulatory environment relaxing to any great extent. Society may be right to prefer a tougher regulator, even if it means more costly R&D. Drug safety matters. And although the 1950s and 1960s may be viewed by some as a golden age in terms of therapeutic innovation[36, 48, 54], it seems unlikely that the severe adverse outcomes for many patients taking part in clinical trials during this period36 would be acceptable today.
The narrow clinical search problem. […] In the 1950s and 1960s, initial screening was typically performed in animals, not in vitro or in silico, and drug candidates were given in early stages of the development process to a range of physicians. Discovery involved, to an extent, the ability of physicians to spot patterns through careful clinical observation, especially in therapeutic areas in which symptomatic improvements are readily observable, such as psychiatry[36, 49, 50, 51]. This is sometimes dismissed as serendipity but the approach made it likely that new therapeutic effects would be detected. Even recently, it appears that many — perhaps most — new therapeutic uses of drugs have been discovered by motivated and observant clinicians working with patients in the real world[72]. Some drug companies, particularly smaller and mid-sized firms, recognize this opportunity and are active repositioners of existing drugs.
However, the ‘cautious regulator’ problem and the ‘basic research–brute force’ bias have pushed most of the drug industry towards a narrow clinical search strategy. If a drug has an effect but this is not the precise effect that the trial designers anticipated, then the trial fails. Opportunities for serendipity are actively engineered out of the system. Perhaps it is too risky to let bright doctors with large numbers of patients make broad clinical observations, or to let creative scientists rummage around in rich clinical data sets, in case they find something unexpected, which has to be explained to the cautious regulator who then kills the project.
[…]
The big clinical trial problem.
[…]
Furthermore, Phase III trials have become a messy mixture of science, regulation, public relations and marketing. Trying to satisfy these multiple constraints tends to inflate their size and cost.
The best clinical trial to show efficacy would be something relatively small in a homogeneous patient sample recruited from as few centres as possible — the medical equivalent of a well-controlled experiment. But this tends to make the cautious regulator uneasy given variation in practice patterns and patients. What about rare side effects (the FDA has recently required post-marketing trials for long-acting bronchodilators in around 53,000 patients)?
[…]
The long cycle time problem. In the 1950s and 1960s, cycle times were remarkably short by modern standards. The regulator was less cautious and there was less molecular reductionism before agents were screened for efficacy in animal models and in patients. This sped up innovation.»
— Scannell JW et al. “Diagnosing the decline in pharmaceutical R&D efficiency.” Nat Rev Drug Discov (2012) vol. 11 (3) pp. 191-200
https://www.nature.com.sci-hub.bz/nrd/journal/v11/n3/full/nrd3681.html
https://www.ncbi.nlm.nih.gov/pubmed/22378269
«Multiple studies have demonstrated that the original directive not only failed to harmonize regulations, but that it brought in an era of stifling bureaucracy and probably contributed to a perception that Europe is not an attractive place to conduct clinical trials. Researchers have voiced concerns that the current system demands large volumes of paperwork, creates difficulties for multinational studies that must operate under different national interpretations of the directive, delays trial initiation and imposes additional costs.»
nature.com/nrd/journal/v11/n9/full/nrd3843.html
«These observations underscore the need for an ongoing assessment of a new drug’s effectiveness and safety in the post-marketing period. Importantly, the preoccupation of the press, the public and even politicians with issues of drug safety is often misplaced, because of a focus on safety alone, even though what determines the value of a drug is its benefit–risk balance[1].
[…]
The development of innovative drugs has become more challenging and more costly in recent decades for several reasons. Regulatory authorities have heightened their requirements for evidence of efficacy and safety (in part in response to major drug safety problems), and cash-strapped payers for health care increasingly need strong evidence that new drugs represent value for money compared with existing drugs. Indeed, industry may have shifted the emphasis of its innovative drug development into areas where the bar for approval is lower owing to a lack or absence of effective drugs, but the risk of failure is higher. Nevertheless, in some disease areas there has been considerable success, such as biologics for patients with rheumatoid arthritis and molecularly targeted drugs for some cancers (such as chronic myeloid leukaemia). However, the costs of failure can be huge, particularly for indications such as Alzheimer’s disease that require lengthy clinical trials and that lack validated surrogate end points.
In the United States, and some European countries, an increasingly vocal patient advocacy movement has demanded earlier access to new medicines, particularly for life-threatening diseases. This movement, which had its origins in AIDS and cancer advocacy in the 1980s, has put regulators under pressure to relax their evidentiary requirements for the approval of potentially life-saving medicines. Most regulatory agencies have responded with accelerated approval tracks and compassionate use programmes but there are some commentators who still press for a broad reversal of a regulatory trend requiring a higher level of evidence for drug approval. For example, a former commissioner of the US Food and Drug Administration (FDA) recently called for the US Congress to allow the approval of new drugs based solely on drug safety, with efficacy to be proven in post-approval trials[2]. The US Congress is currently considering legislation to speed market access of drugs for serious diseases by allowing the expanded use of surrogate end points and rapidly measurable clinical end points and thus, presumably, drug approval based on fewer, smaller and shorter trials.»
nature.com/nrd/journal/v11/n7/full/nrd3787.html
«Driven by increasing regulatory challenges and costs of getting a mass-market drug approved, industry has been moving from a blockbuster model towards ‘niche-buster’ opportunities. Typically, orphan drugs command premium pricing, and thus generate significant revenues even in small markets, thereby making them attractive to the industry. Although early on the majority of approved orphan drugs were developed in biotech companies, big pharma has been responsible for a growing number of approvals — from 35% in 2000–2002 to 56% in 2006–2008. In fact, globally, large pharmaceutical companies account for over 50% of the orphan drug market4. More recently, several pharmaceutical companies, such as Pfizer and GlaxoSmithKline, have formally embraced the orphan drug model by unveiling strategies for treating rare diseases through partnerships and acquisitions[3]. The expected growth in the number of orphan treatments, although beneficial for patients, could eventually put additional economic burden on the already strained health-care system[1, 2, 4].»
nature.com/nrd/journal/v11/n4/full/nrd3654.html
• Steve Blank. “Reinventing Life Science Startups: Therapeutics and Diagnostics.” (August 19, 2013)
steveblank.com/2013/08/19/reinventing-life-science-startups-evidence-based-entrepreneurship
“Medical Devices and Digital Health.” (August 20, 2013)
steveblank.com/2013/08/20/reinventing-life-science-startups-medical-devices-and-digital-health
“Evidence-based Entrepreneurship.” (August 21, 2013)
steveblank.com/2013/08/21/reinventing-life-science-startups-evidence-based-entrepreneurship-2
• “Kefauver-Harris Amendments Revolutionized Drug Development.” FDA Consumer Health Information (October 10, 2012)
https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm322856.htm
URL G+ post with further references and excerpts:
plus.google.com/+ZephyrLópezCervilla/posts/1ip9zfajqux
3. On how reliable and useful state-funded research is (basic or otherwise):
• Phil Davis. “Most NIH-Sponsored Trials Slow to Publish, Many Aren’t Published, Most Fail to Report Data, Studies Show.” The Scholarly Kitchen (February 21, 2012)
https://scholarlykitchen.sspnet.org/2012/02/21/reporting-of-nih-sponsored-trials
• Nicholson JM and Ioannidis, JPA. “Research grants: Conform and be funded.” Nature (2012) vol. 492 (7427) pp. 34-36
http://nature.com.sci-hub.bz/nature/journal/v492/n7427/full/492034a.html
https://www.ncbi.nlm.nih.gov/pubmed/23222591
Excerpt:
plus.google.com/+ZephyrLópezCervilla/posts/DFFbzMsFmr1
Graphics:
nature.com/nature/journal/v492/n7427/images/492034a-i2.0.jpg
Supplementary Information:
nature.com/nature/journal/v492/n7427/extref/492034a-s1.pdf
• David H Freedman. “Lies, Damned Lies, and Medical Science.” The Atlantic (November, 2010)
theatlantic.com/magazine/archive/2010/11/lies-damned-lies-and-medical-science/308269
• Ioannidis JP. “Why most published research findings are false.” PLoS Med (2005 Aug) vol. 2 (8) pp. e124
http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0020124
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1182327
https://www.ncbi.nlm.nih.gov/pubmed/16060722
• Bob Yirka. “Research duo say that far too many preclinical cancer study results are just plain wrong.” Medical Xpress (March 29, 2012)
https://medicalxpress.com/news/2012-03-duo-preclinical-cancer-results-plain.html
• Sharon Begley. “In cancer science, many ‘discoveries’ don’t hold up.” Reuters (March 27, 2012)
http://www.reuters.com/article/us-science-cancer-idUSBRE82R12P20120328
• Prinz F, Schlange T and Asadullah K. “Believe it or not: how much can we rely on published data on potential drug targets?” Nat Rev Drug Discov (2011 Aug 31) vol. 10 (9) p. 712
http://www.nature.com/nrd/journal/v10/n9/full/nrd3439-c1.html
https://www.ncbi.nlm.nih.gov/pubmed/21892149
• Ellis LM and Begley CG. “Drug development: Raise standards for preclinical cancer research.” Nature (2012 Mar 28) vol. 483 (7391) pp. 531-3
http://www.nature.com/nature/journal/v483/n7391/full/483531a.html
https://www.ncbi.nlm.nih.gov/pubmed/22460880
URL G+ post with further references and excerpts:
plus.google.com/+ZephyrLópezCervilla/posts/gnMSZQMwKFz
plus.google.com/+ZephyrLópezCervilla/posts/G4xPWeykkdx
plus.google.com/+ZephyrLópezCervilla/posts/iW2cNjsmmnq
4. On the legislative hurdle imposed by the “benefactor legislator”:
A number of relevant drugs were discovered and developed without the “need” of patents:
«Fleming discovered penicillin in 1928 and started to study its antibiotic properties. It was to be 15 years before penicillin would be produced in commercial quantities.
[…]
He did not patent his discovery, preferring that all would benefit from it.»
http://www.sath.org.uk/edscot/www.educationscotland.gov.uk/scotlandshistory/20thand21stcenturies/alexanderfleming/index.html
Fleming discovered penicillin while working at St. Mary’s Hospital in London, at that time still a private institution (some sort of charity foundation):
«In 1948 it joined the NHS under the control of the Paddington Group Hospital Medical Committee, part of the North West Metropolitan Regional Health Board.»
http://ezitis.myzen.co.uk/stmarysharrowroad.html
«St Mary’s Paddington, the first institution to be conceived from the start as a teaching hospital with a medical school attached, was founded in 1851 from small philanthropic beginnings based on ‘Christian and genteel values’, in an area teeming with sailors and prostitutes.»
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1079568
«St Mary’s Hospital first opened its doors to patients in 1851, the last of the great voluntary hospitals to be founded.»
https://en.wikipedia.org/wiki/St_Mary's_Hospital,_London
https://en.wikipedia.org/wiki/History_of_the_National_Health_Service_(England)
«Voluntary hospitals were independent charities established for the benefit of the ‘deserving poor’, who, according to Victorian values, were respectable working-class people who had fallen on hard times because of sickness.»
https://www.imperial.nhs.uk/about-us/who-we-are/our-history
«Moreover, for the limited utility that Fleming wrote about in his papers, there was little reason to patent penicillin. In any event, the patent would nearly have expired by the time Florey and Chain did their crucial clinical experiments in mice and then humans in 1941, and would have had only a few years left when production was sufficient to lead to broader clinical use in 1944 and 1945. Moreover, the inducement to produce penicillin during World War II was largely driven by the War Production Board, and far from encouraging proprietary exclusive property rights, the U.S. Government basically forced various pharmaceutical manufacturers to share technology, including various manufacturing patents. It is crystal clear that the missing element in the 1928 to 1941 gap was not absence of patent incentive.»
— N Stephan Kinsella. “Patent and Penicillin.” Mises Economic Blog (June 22, 2006)
https://mises.org/blog/patent-and-penicillin
URL G+ post with further references and excerpts:
plus.google.com/+ZephyrLópezCervilla/posts/6T5qpFyLAMH
plus.google.com/+ZephyrLópezCervilla/posts/1ip9zfajqux
• Michele Boldrin and David K Levine. “Against Intellectual Monopoly.” Cambridge University Press (2008)
http://levine.sscnet.ucla.edu/papers/imbookfinalall.pdf
Excerpt: plus.google.com/114605547533973731226/posts/aPA3xZEQpHF
• James Bessen and Michael J Meurer. “Patent Failure: How Judges, Bureaucrats, and Lawyers Put Innovators at Risk.” Princeton University Press (March 2008)
http://emilkirkegaard.dk/en/wp-content/uploads/James-Bessen-and-Michael-J.-Meurer-Patent-Failure-How-Judges-Bureaucrats-and-Lawyers-Put-Innovators-at-Risk.pdf
http://www.doc88.com/p-341802802298.html
• Boldrin, Michelle and Levine, David K. “The Case Against Patents.” Federal Reserve Bank of St. Louis Working Paper Series (2012) 2012-035A
https://research.stlouisfed.org/wp/2012/2012-035.pdf
• Hansen, Stephen A et al. “The Effects of Patenting AAAS in the Scientific Community.” American Association for the Advancement of Science (2006)
http://wipo.int/ip-outreach/en/tools/research/details.jsp?id=174
• Hansen, Stephen A et al. “Intellectual Property Experiences in the United States Scientific Community.” American Association for the Advancement of Science (2007)
http://w.astro.berkeley.edu/~kalas/ethics/documents/intellectual_property/SIPPI_US_IP_Survey.pdf
• Ritter DS. “Switzerland’s Patent Law History.” Fordham Intell. Prop. Media & Ent. L.J. (2004) vol. 14 pp. 463-496
http://ir.lawnet.fordham.edu/cgi/viewcontent.cgi?article=1419&context=iplj
• Michael Fitzgerald. “A Patent Is Worth Having, Right? Well, Maybe Not.” The New York Times (July 15, 2007)
http://www.nytimes.com/2007/07/15/business/yourmoney/15proto.html
• Sheldon Richman. “Patent Nonsense.” The American Conservative (January 18, 2012)
http://www.theamericanconservative.com/articles/patent-nonsense
• Jordan Weissmann. “The Case for Abolishing Patents (Yes, All of Them).” The Atlantic (September 27, 2012)
http://www.theatlantic.com/business/archive/2012/09/the-case-for-abolishing-patents-yes-all-of-them/262913
URL G+ post with further references and excerpts:
plus.google.com/+ZephyrLópezCervilla/posts/aPA3xZEQpHF
plus.google.com/+ZephyrLópezCervilla/posts/HdAVwzabfg5
5. On the effect of the welfare state on the funding of scientific research:
«The growth of the welfare state is approaching the stage where virtually the only money available for scientific research will be government money. (The disastrous effects of this situation and the disgraceful state of government-sponsored science are apparent already, but that is a different subject. We are concerned here only with the moral dilemma of scientists.) Taxation is destroying private resources, while government money is flooding and taking over the field of research.
In these conditions, a scientist is morally justified in accepting government grants—so long as he opposes all forms of welfare statism. As in the case of scholarship-recipients, a scientist does not have to add self-martyrdom to the injustices he suffers.»
— Ayn Rand. “The Question of Scholarships.” The Objectivist (June 1966) no. 11
https://books.google.com/books?id=OsCSArJxIRwC&pg=PT47
Excerpt: http://aynrandlexicon.com/lexicon/government_grants_and_scholarships.html
URL G+ post with further references and excerpts:
plus.google.com/+ZephyrLópezCervilla/posts/i5ydjx2jWxS
]]>LOVELYPINK _NIZA hey
]]>Zephyr López Cervilla
From your post I got 2 things. You know how to copy/paste(good for you), and you didn’t understand what I said at all.
I did not say that the government funds the majority of the cost to research drugs. I said the government funds the majority of the early research of drugs. Once the drug looks promising the drug companies fund almost all of it(minus the insane amount them taking those costs off their taxes saves them of course). So in the end, the drug companies pay for the majority of the research costs of a drug. But that doesn’t change the truth of what I said. Which was limited to the early stages, which I made clear in my comment.
And I’m not sure why you bothered mentioning Google. The federal government spends almost 10 times more on science than Google even grosses in a year.
And you need to find better sources to research from. That “this growth of the welfare state” comment is pure horseshit. Government funding of science isn’t growing, it’s falling. Which makes the rest of the comment equally horseshit.
]]>ขชขขจชขขขยจจขขขชชขชขขยชขชชขชชชชชชชนยยสสขาข.าา.าื
]]>vipi
]]>